It seems that there is a lot of confusion as to what isotopes, radioisotopes, nuclides, and radionuclides are. First, we have to go back to chemistry class and remember the periodic table of elements, which lists all of the chemical elements in an organized fashion.
The periodic table reports each element with its average properties. Each chemical element on the periodic table has a distinct number of protons. The reason we say “average properties” here is because each element has a number of different isotopes. The word “isotope” indicates an equal number of protons, hence the prefix “iso” and the letter “p” in the name (note that isotones represent nuclides with the same number of neutrons). For example, hydrogen (1 proton) consists of 3 natural isotopes: hydrogen (0 neutrons), deuterium (1 neutron), and tritium (2 neutrons). The same is true of uranium, where U-235 is an isotope that can undergo fission. The number 235 represents the sum of neutrons and protons that make up the nucleus of the uranium atom (92 protons and 143 neutrons). The term “nuclide” is just a general name for any isotope of a chemical element.
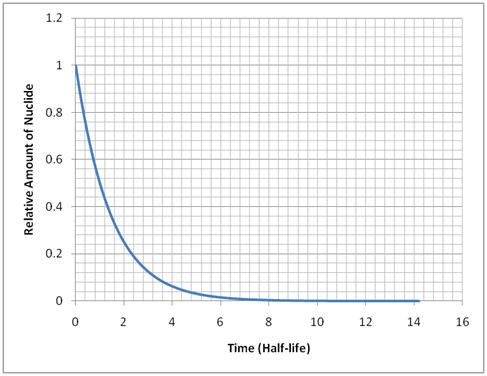
The prefix “radio” in front of “isotope” and “nuclide” refers to radioactivity. This indicates the spontaneous transformation (decay) of unstable nuclides to more stable ones. In order to accomplish this, nuclides may emit a spectrum of particles including alpha particles, beta particles (electrons or positrons), neutrons, gamma rays (photons), or x-rays. In order to characterize the probability of a nuclide decaying, each radionuclide has a half-life. The half-life of a radionuclide is the expected time it takes for one half of the amount of one isotope to decay into another isotope. In terms of radiation safety, it is desirable for unstable nuclides to eventually decay to stable nuclides. The amount of radionuclide present, when there is no source producing it, undergoes an exponential rate of decay.
Activity is another term that is used when talking about radioisotopes. Activity, measured in the unit of Bequerel (Bq), is the number of decays occurring per unit time. It is not necessarily equal to the rate at which particles are emitted. For example, cobalt-60 emits both beta and gamma radiation each time it decays. The activity of an isotope also follows a similar exponential trend as shown above. It is also often expressed in units of Curie (Ci), where 1 Ci = 3.7 x 1010 Bq.
There is also a big difference between nuclear reactions and chemical reactions. Nuclear reactions are quite different for different isotopes of the same element, while chemical reactions are quite similar for different isotopes of the same element. All isotopes of the same element (I-127, I-131, and I-135 are all isotopes of iodine) have similar chemical interactions, but they could result in different health effects due to different levels of radioactivity. This is because chemical reactions involve changing electron configurations in the atom. Since all isotopes of a given chemical element have the same electron configuration, they will have similar chemical reactions. A good example is the use of iodine tablets. Different isotopes of iodine will have similar chemical interactions in the body. Therefore, if the body is already saturated with non-radioactive iodine, it is already full and radioactive iodine has a lower chance of being absorbed. For nuclear reactions, each isotope of an element will have different nuclear reaction characteristics. For example, slow neutrons have a much higher chance of causing fission in U-235 than in U-238.